Global Construction Prime Power Generators Market 2025-2032
October 1, 2025
Global biologics cdmo Market 2026-2034
$4195 – $5695
| Single User | PDF Report, PDF Report + Dashboard (Multi-Users Access), PDF Report + Dashboard (Single User Access) |
|---|
Report Summary
Revolutionize the way you engage with data through our cutting-edge interactive dashboard(Click to enlarge)
- The global biologics CDMO market in 2026 is defined by a strategic pivot from standardized manufacturing toward a partnership-led, high-complexity service model. As pharmaceutical portfolios increasingly migrate from traditional monoclonal antibodies toward sophisticated modalities—such as antibody-drug conjugates (ADCs), bispecifics, and GLP-1 agonists—the role of the CDMO has transitioned from a tactical capacity provider to a fundamental architect of the drug’s lifecycle. This evolution is underscored by the integration of AI-driven bioprocessing and digital twins to optimize yields and compress the “path-to-clinic,” allowing sponsors to mitigate the high capital risks associated with large-molecule development while accessing specialized technical expertise that is increasingly difficult to maintain in-house.
- Simultaneously, the market is navigating a complex geopolitical and operational landscape where supply chain resilience is a primary competitive differentiator. Heightened regulatory scrutiny and initiatives aimed at diversifying manufacturing footprints—such as the onshoring of critical biological assets—have spurred significant infrastructure investments in North America and Europe, even as Asia-Pacific matures into a sophisticated hub for high-volume commercial production. To capture value in this environment, leading CDMOs are adopting “one-stop-shop” integrated platforms that bridge the gap between early-stage development and sterile fill-finish. This consolidation of services, combined with the adoption of sustainable, single-use technologies and continuous manufacturing, ensures that providers can offer the agility required to support both the specialized needs of lean biotech firms and the global scale required by established pharmaceutical giants.
Table of Content
1. Report Scope
1.1. Market Segmentation and scope
1.2. Regional Scope
1.3. Estimates and forecast timeline
2. Market Research Methodology
2.1. Research methodology and design
2.2. Sample selection
2.3. Reliability and validity
3. Executive Summary
4. Market Analysis
4.1. Market size and growth rates
4.2. Market growth drivers, market dynamics and trends
4.3. Market scenarios and opportunity forecasts
4.4. Market constraints and challenges
4.5. Industry value chain analysis
4.6. Industry analysis – Porter’s
4.6.1. Threat of new entrants
4.6.2. Bargaining power of suppliers
4.6.3. Bargaining power of buyers
4.6.4. Threat of substitutes
4.6.5. Competitive rivalry
4.7. PEST analysis
4.7.1. Political/legal landscape
4.7.2. Economic landscape
4.7.3. Social landscape
4.7.4. Technological landscape
5. Market Breakdown – by Modality
5.1. Introduction
5.2. Monoclonal Antibodies (mAbs)
5.3. Cell and Gene Therapies (CGT)
5.4. Antibody-Drug Conjugates (ADCs)
5.5. Recombinant Proteins
5.6. Vaccines (mRNA, Viral Vector, Subunit)
5.7. Others (Peptides, Nucleic Acids)
6. Market Breakdown – by Workflow
6.1. Introduction
6.2. Clinical
6.3. Commercial
7. Market Breakdown – by Expression System
7.1. Introduction
7.2. Mammalian Systems
7.3. Microbial Systems
7.4. Others
8. Market Breakdown – by Service Type
8.1. Introduction
8.2. Contract Development (Cell Line & Process Development)
8.3. Drug Substance Manufacturing (API)
8.4. Drug Product Manufacturing (Fill-Finish)
8.5. Analytical & Quality Control Services
9. Market Breakdown – by Therapeutic Area
9.1. Introduction
9.2. Oncology
9.3. Autoimmune Diseases
9.4. Infectious Diseases
9.5. Metabolic Diseases
9.6. Cardiovascular Diseases
9.7. Neurological Diseases
9.8. Others
10. Market Breakdown – by End-Use
10.1. Introduction
10.2. Pharmaceutical Companies
10.3. Biotechnology Companies
10.4. Academic and Research Institutions
10.5. Others
11. Market Breakdown – by Geography
11.1. North America
11.1.1. North America Biologics CDMO Market, 2026-2034
11.1.2. North America Biologics CDMO Market, by Modality
11.1.3. North America Biologics CDMO Market, by Workflow
11.1.4. North America Biologics CDMO Market, by Expression System
11.1.5. North America Biologics CDMO Market, by Service Type
11.1.6. North America Biologics CDMO Market, by Therapeutic Area
11.1.7. North America Biologics CDMO Market, by End-Use
11.1.8. North America Biologics CDMO Market, by Country
11.1.8.1. U.S.
11.1.8.2. Canada
11.1.8.3. Mexico
11.2. South America
11.2.1. North America Biologics CDMO Market, by Modality
11.2.2. North America Biologics CDMO Market, by Workflow
11.2.3. North America Biologics CDMO Market, by Expression System
11.2.4. North America Biologics CDMO Market, by Service Type
11.2.5. North America Biologics CDMO Market, by Therapeutic Area
11.2.6. North America Biologics CDMO Market, by End-Use
11.2.7. North America Biologics CDMO Market, by Country
11.2.7.1. Brazil
11.2.7.2. Argentina
11.2.7.3. Others
11.3. Europe
11.3.1. North America Biologics CDMO Market, by Modality
11.3.2. North America Biologics CDMO Market, by Workflow
11.3.3. North America Biologics CDMO Market, by Expression System
11.3.4. North America Biologics CDMO Market, by Service Type
11.3.5. North America Biologics CDMO Market, by Therapeutic Area
11.3.6. North America Biologics CDMO Market, by End-Use
11.3.7. North America Biologics CDMO Market, by Country
11.3.7.1. Germany
11.3.7.2. France
11.3.7.3. U.K.
11.3.7.4. Italy
11.3.7.5. Spain
11.3.7.6. Sweden
11.3.7.7. Switzerland
11.3.7.8. Netherlands
11.3.7.9. Russia
11.3.7.10. Others
11.4. Asia-Pacific
11.4.1. North America Biologics CDMO Market, by Modality
11.4.2. North America Biologics CDMO Market, by Workflow
11.4.3. North America Biologics CDMO Market, by Expression System
11.4.4. North America Biologics CDMO Market, by Service Type
11.4.5. North America Biologics CDMO Market, by Therapeutic Area
11.4.6. North America Biologics CDMO Market, by End-Use
11.4.7. North America Biologics CDMO Market, by Country
11.4.7.1. China
11.4.7.2. Japan
11.4.7.3. South Korea
11.4.7.4. India
11.4.7.5. Australia
11.4.7.6. Others
11.5. Middle East & Africa
11.5.1. North America Biologics CDMO Market, by Modality
11.5.2. North America Biologics CDMO Market, by Workflow
11.5.3. North America Biologics CDMO Market, by Expression System
11.5.4. North America Biologics CDMO Market, by Service Type
11.5.5. North America Biologics CDMO Market, by Therapeutic Area
11.5.6. North America Biologics CDMO Market, by End-Use
11.5.7. North America Biologics CDMO Market, by Country
11.5.7.1. Saudi Arabia
11.5.7.2. UAE
11.5.7.3. Israel
11.5.7.4. South Africa
11.5.7.5. Others
12. Competitive Landscape
12.1. Market Competitive Scope & Methodology
12.2. Phase-Based Coverage Landscape
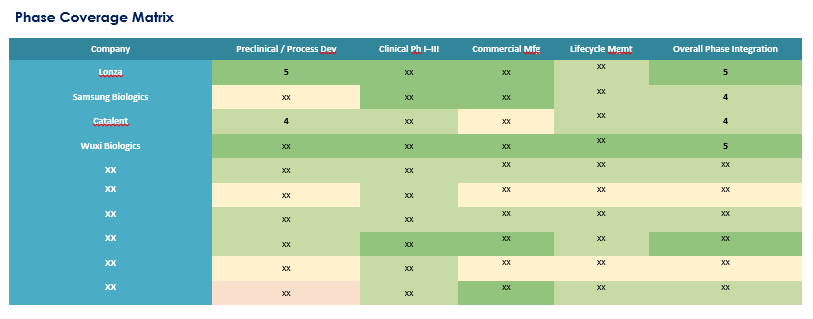
12.3. Phase Coverage Matrix
12.4. Capability Benchmarking
12.5. Capability × Region Overlay
12.6. Aggregated Competitive Heatmap
12.7. Portfolio Breadth vs Depth Analysis
12.8. Phase Transition Continuity Assessment
12.9. Strategic Positioning Summary
12.10. Quantitative Competitive Scorecard
12.11. White-Space Opportunity Map
12.12. Competitive Implications & Strategic Takeaways
12.13. Global Revenue Share Analysis (%), by Leading Players
12.14. North America Revenue Share Analysis (%), by Leading Players
12.15. Europe Revenue Share Analysis (%), by Leading Players
12.16. APAC Revenue Share Analysis (%), by Leading Players
12.17. South America Revenue Share Analysis (%), by Leading Players
12.18. MEA Revenue Share Analysis (%), by Leading Players
12.19. Competitive Universe Overview
12.19.1. 3P Biopharmaceuticals
12.19.2. 53Biologics
12.19.3. AbbVie Contract Manufacturing
12.19.4. Adragos Pharma
12.19.5. AGC Biologics
12.19.6. Aenova Group
12.19.7. Ajinomoto Bio-Pharma Services (Althea)
12.19.8. Almac Group
12.19.9. Aragen Bioscience
12.19.10. Axplora (incl. Novasep)
12.19.11. Bachem
12.19.12. Baxter BioPharma Solutions
12.19.13. Biomay
12.19.14. Biovian Oy
12.19.15. Binex Co., Ltd.
12.19.16. BiosanaPharma
12.19.17. Boehringer Ingelheim International GmbH (BioXcellence)
12.19.18. Cambrex (Emerging in biologics)
12.19.19. Catalent Biologics
12.19.20. Cenexi – Laboratoires Thissen SA
12.19.21. Celonic Group
12.19.22. Charles River Laboratories (Biologics CDMO services)
12.19.23. Cobra Biologics (Charles River)
12.19.24. CordenPharma
12.19.25. Curia Global (formerly AMRI)
12.19.26. Cytiva (bioprocess/biologics services)
12.19.27. Delpharm
12.19.28. Emergent BioSolutions (CDMO division)
12.19.29. Eurofins CDMO
12.19.30. Evonik (biologics/advanced drug delivery CDMO)
12.19.31. Famar SA
12.19.32. Fareva
12.19.33. FUJIFILM Diosynth Biotechnologies
12.19.34. IDT Biologika GmbH
12.19.35. Jubilant Biosys / KBI Biopharma
12.19.36. Lonza Group AG
12.19.37. Merck KGaA / MilliporeSigma CDMO
12.19.38. MGI / Mabion (EU biologics CDMOs)
12.19.39. Minaris Regenerative Medicine
12.19.40. Novartis / Sandoz Contract Manufacturing
12.19.41. Oxford Biomedica
12.19.42. Patheon (Thermo Fisher Scientific)
12.19.43. Pelican BioTherapeutics
12.19.44. Pfizer CentreOne
12.19.45. Pierre Fabre CDMO
12.19.46. Polpharma Biologics
12.19.47. Polymun Scientific
12.19.48. Porton Pharma Solutions (Porton Bio)
12.19.49. Prothya Biosolutions
12.19.50. Recipharm AB
12.19.51. Rentschler Biopharma SE
12.19.52. Richter Biologics
12.19.53. Samsung Biologics
12.19.54. Samsung Bioepis (contract operations subset)
12.19.55. Siegfried Holding AG
12.19.56. Thermo Fisher Scientific (Patheon)
12.19.57. Toho / Kyowa Kirin (biologics CDMO units)
12.19.58. Vetter Pharma International
12.19.59. WuXi Biologics (incl. EU sites in IE & DE)
12.19.60. Zelluna / other emerging EU CGT CDMOs